Newsroom
Science Update
Researchers Reveal a Novel Role of AGO Proteins in Regulating Protein Quality Control on ER
Mar 23, 2022
The miRNA-mediated gene silencing and the ubiquitin-mediated protein quality control represent two fundamental mechanisms for controlling gene expression. However, little is about how these two processes are coupled to regulate gene expression. In the recent issue of Molecular Cell (11 a.m. ET on 23 March, 2022), Qinmiao Sun lab from Institute of Stem Cell and Regeneration of the Chinese Academy of Sciences and Dahua Chen lab from Yunnan University discovered that Argonaute proteins (AGOs) on the ER play a novel role in regulating protein quality control via lipid-mediated phase separation, substantially coupling post-transcriptional gene silencing and protein quality control processes to ensure efficient gene silencing.
AGOs are well-known to silence target gene expression by interacting with miRNAs and involved in regulating a wide variety of biological processes. In addition to their cytoplasmic localization, AGOs have also been reported to associate with membrane-bound organelles including ER. However, the functional role of AGO proteins' association with the ER membrane and how this association is regulated have been unknown.
In this study, the researchers discovered a conserved lipid-binding motif in the N domain of AGO proteins that specifically interacts with PI(4, 5)P2 and the AGO-PI(4, 5)P2 interaction enhanced the phase separation of AGOs on the ER. Moreover, they found that the ER-localized AGO condensates recruits Ltn1 to catalyze nascent-peptide ubiquitination, subsequently leading to degradation of the unwanted protein products. These findings suggest that AGOs on the ER can couple two fundamental cellular processes, post-transcriptional gene silencing and protein quality control, to ensure proper gene expression.
Given that the human AGO2 overexpression is related to tumorigenesis and cancer aggressiveness and that its lipid-binding motif contains two cancer-related mutations (based on COSMIC database), it will be of great interest to investigate whether the lipid-mediated membrane function of human AGO2 is related to cancer development in the future.
This study entitled "Lipid-mediated phase separation of AGO proteins on the ER controls nascent-peptide ubiquitination" were published in Molecular Cell.
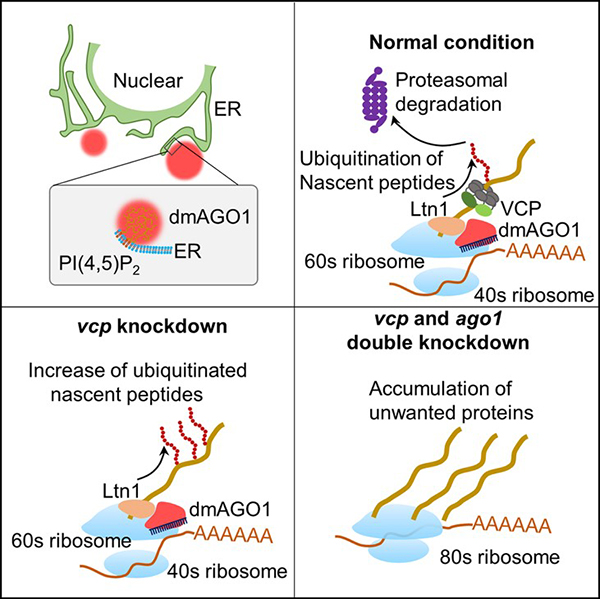
The mechanism of lipid-mediated phase separation of AGO proteins on the ER in regulating nascent-peptide ubiquitination (Image by Chen's lab)
https://www.sciencedirect.com/science/article/pii/S109727652200209X
(11 a.m. ET on 23 March, 2022)
Contact:
Qinmiao Sun
Institute of Zoology Chinese Academy of Sciences
Tel:86-64807391
E-mail: qinmiaosun@ioz.ac.cn
Web: http://english.ioz.cas.cn/
附件:

