Newsroom
Science Update
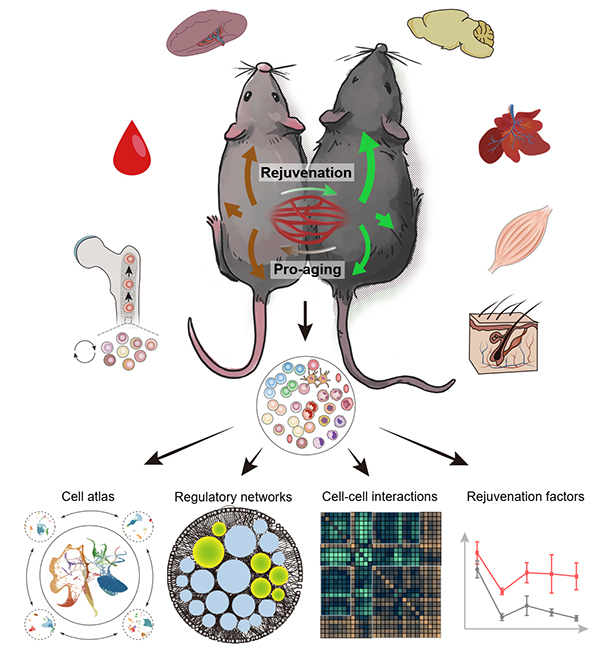
Old Mice, Young Milieu: Understanding How Aged Stem Cells Are Revitalized for Systemic Rejuvenation
In a recent study published in Cell Stem Cell, researchers from the Institute of Zoology and the Beijing Institute of Genomics of the Chinese Academy of Sciences probed the effects of HP in mice at the single-cell level, generating a systemic atlas of HP and aging.
Jun 01, 2022
Aging is a process of systemic degeneration involving a variety of tissues and organs in the whole body and is characterized by gradual reduction of regenerative ability and functional decline. In trying to understand the aging process, and more importantly, in pursuit to reverse it, scientists have developed a technique called heterochronic parabiosis (HP), which surgically connects the circulatory systems of a young and old animal, thus providing a unique paradigm for evaluating how tissues and organs respond to the opposite milieu at a systemic level.
Using HP, the revitalizing power of young blood was demonstrated in the late 1950s and early 1960s. Since the early 2000s, interest in HP has revived. Despite this renewed interest, though, how young blood makes aged bodies return to a “younger state” has so far remained a mystery.
In a recent study published in Cell Stem Cell, researchers from the Institute of Zoology and the Beijing Institute of Genomics of the Chinese Academy of Sciences probed the effects of HP in mice at the single-cell level, generating a systemic atlas of HP and aging.
With this atlas in hand and followed by functional validations, the researchers revealed cellular and molecular changes in the aged and young parabionts at single-cell resolution. More importantly, they revealed key mediators of the systemic effect and their cellular targets. This information may eventually provide important clues for the development of aging biomarkers in humans as well as new therapeutics for aging intervention.
According to the researchers, exposure to old blood can accelerate aging of various organs/tissues and cell types in the young parabiont, while reciprocally, exposure to young blood can elicit rejuvenation in the aged parabiont, especially through targeting the stem/progenitor cells and their niches in aged tissues across systems.
Adult stem cells continuously self-renew to maintain the somatic cell population and repair age-related damage. These activities are actively supported by neighboring or niche cells, and instructed by systemic factors that elicit intrinsic alterations directly in the stem cells or the niche environment.
The researchers determined that the activated stem/progenitor cell population includes basal stem cells and hair follicle stem cells (HFSCs) in skin; fibroblast/adipose progenitor cells (FAPs) in skeletal muscle; and hematopoietic stem and progenitor cells (HSPCs) located in bone marrow.
Aged HSPCs are among the cells most sensitive to young blood, whose exposure switches the transcription regulation network of HSPCs to a younger state, thus regaining the potential for lymphoid differentiation and restoring the population of lymphoid cells (such as pro-B cells) in aged bone marrow.
Notably, using a sophisticated CD45 congenic system to trace cell origins in parabionts, researchers verified that the rejuvenation effects were due to activation of aged HSPCs rather than relocation of young HSPCs from the other parabiont into the bone marrow. This discovery has clarified a puzzling issue by demonstrating a certain degree of cellular compartmentalization despite a conjoined circulatory system.
Moreover, the researchers identified a series of HSPC aging regulators including epigenetic regulatory factors such as YY1 and chemokines such as CCL3. For validation, researchers used mouse transplantation experiments to show that lentivirus-mediated YY1 overexpression could effectively enhance the reconstructive ability of aged HSPCs while similar “gene therapy” based on CCL3 overexpression could significantly improve the ability of aged HSPCs to differentiate into lymphoid cells.
In addition, several candidate targets, especially in pathways that reduce inflammation and restore cellular communication, were also identified, thus providing new rejuvenation strategies for solid tissues.
All in all, this multi-organ, multi-dimensional study reveals various key factors regulating the "rejuvenation" of adult stem cells and surrounding somatic cells, providing rich data resources for the establishment of new biomarkers and intervention strategies for aging. This work thus provides a new paradigm for discovering the “youth factors” from the perspective of systems biology.
Figure 1. Systematic biology study on how young blood promote the rejuvenation of multiple tissues and organs (Image from Ma et al. Cell Stem Cell 2022)
Original link: https://doi.org/10.1016/j.stem.2022.04.017
Contact:
Guang-Hui Liu
Institute of Zoology Chinese Academy of Sciences
Tel: 86-64807852
E-mail: ghliu@ioz.ac.cn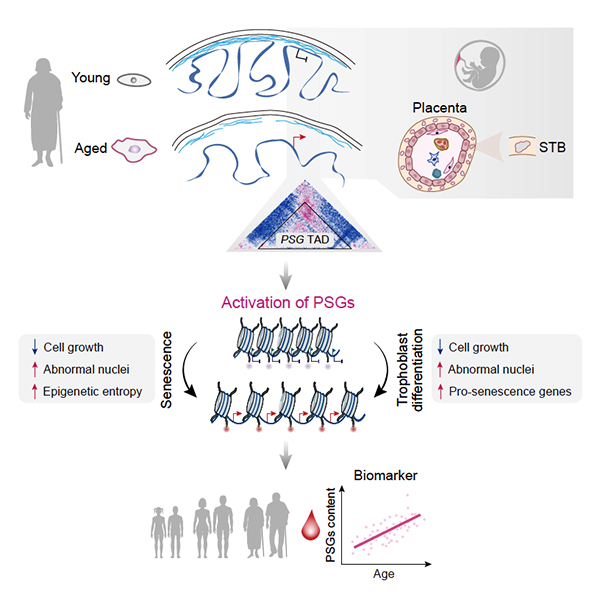
Increase in Chromatin Entropy Drives Cellular Aging, Say Researchers
In a new study published on Developmental Cell, researchers from the Institute of Zoology and the Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to decode the large-scale remodeling of the epigenomic landscape during aging. They found that aberrant expression of placenta-related genes is the key driver and molecular biomarker of cellular aging.
Jun 01, 2022
At the center of every cell, highly organized chromatin encodes the program of life with just one set of genes. This is possible because different genes are activated at different stages of life while remaining silent otherwise. Some genes for previous stage of life are buried deep at the nuclear periphery, while some manage to escape from repression.
Researchers have been deciphering this hierarchical organization and how it keeps everything in order. However, a new study shows that the system turns chaotic during aging. Specifically, chaos emanates from the nuclear periphery, resulting in large-scale chromatin reorganization and epigenetic dysregulation that ultimately drives cellular senescence.
In a new study published on Developmental Cell, researchers from the Institute of Zoology and the Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to decode the large-scale remodeling of the epigenomic landscape during aging. They found that aberrant expression of placenta-related genes is the key driver and molecular biomarker of cellular aging.
Aging is associated with stem cell exhaustion, the hallmarks of which include a diverse spectrum of epigenetic dysregulation. At the cellular level, aged human stem cells frequently have deformed nuclei. But how this aging phenotype relates to the aged epigenetic state was not well understood.
Using isogenic young and senescent, progeroid human mesenchymal progenitor cells (hMPCs) as a cellular aging paradigm, the researchers generated a large-scale, high-resolution epigenomic landscape resource. Through in-depth analyses of reorganization and interplay within and in between hierarchical layers of epigenomic organization during the course of hMPC senescence, the researchers detected large-scale alterations caused by global relaxation of structural constraints in the nuclear lamina and loss of epigenetic markers.
They found that the epigenome of senescent cells exhibited increased epigenetic “entropy,” as well as compromised compartmentalization.
In addition to clarifying the general relationship between 3D genomic reorganization and transcriptional dysregulation, they identified specific topological conversions that lead to ectopic expression of developmentally restricted genes, e.g., a cluster of pregnancy-specific glycoprotein (PSG) genes.
They verified that dissociation of the PSG cluster from the nuclear lamina and consequent reactivation can trigger cellular senescence in aged hMPCs. Moreover, evidence that PSGs serve as novel biomarkers of human organismal aging and are even potential drivers of multiple types of cellular and tissue aging were provided.
All in all, the researchers identified the increased epigenetic entropy and activation of developmentally restricted genes as novel hallmarks and driving forces of human cellular aging. These findings provide novel insights into the epigenomic basis of aging regulation and will guide the development of clinical interventions that can slow aging and alleviate aging-associated disorders.
Figure 1. Aberrant activation of developmentally restricted genes caused by large-scale epigenome remodeling is a driver of human stem cell aging (Image from Liu et al. Cell Stem Cell 2022)
Original link::https://doi.org/10.1016/j.devcel.2022.05.004
Contact:
Guang-Hui Liu
Institute of Zoology Chinese Academy of Sciences
Tel: 86-64807852
E-mail: ghliu@ioz.ac.cn

APOE destabilizes heterochromatin and drives senescence
Prof. Guang-hui Liu and his colleagues from the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences and the Beijing Institute of Genomics, Chinese Academy of Sciences, have collaborated to reveal a novel role of APOE in destabilizing heterochromatin and driving senescence in human stem cells, which was published in Nature Aging on March 28th, 2022.
Mar 29, 2022
Prof. Guang-hui Liu and his colleagues from the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences and the Beijing Institute of Genomics, Chinese Academy of Sciences, have collaborated to reveal a novel role of APOE in destabilizing heterochromatin and driving senescence in human stem cells, which was published in Nature Aging on March 28th, 2022.
APOE is well known as a classical lipid-transport protein that mediates lipid transport. Despite of accumulating evidence showing that it is closely implicated in Alzheimer's disease, atherosclerosis, and human longevity, the role of APOE in the regulation of aging is largely unknown.
The researchers found that the expression of APOE protein increased in aging human stem cells and that human stem cell senescence was accelerated upon APOE overexpression and attenuated by APOE knockout.
Mechanistically, the researchers revealed that nuclear-localized APOE interacted with the nuclear envelope and heterochromatin-associated proteins to promote their degradation via the autophagy-lysosomal pathway, thus facilitating heterochromatin destabilization and contributing to human stem cell senescence.
Last but not least, the researchers also discovered that knockdown of APOE delayed cellular senescence in multiple human cell models.
For the first time, this study uncovers a role of APOE as an epigenetic mediator, expanding our understanding of an entirely new function of APOE in mediating senescence. Collectively, this study provides a potential target with novel mechanistic insights for the development of therapeutic strategies against aging and aging-related disorders.
Figure. Nuclear-localized APOE promotes human stem cell senescence (Image courtesy of Guang-Hui Liu's lab)
Link: https://www.nature.com/articles/s43587-022-00186-z
28 March 2022 at 16:00 (London time), 28 March 2022 at 11:00 (US Eastern Time)
Contact:
Guang-Hui Liu
Institute of Zoology Chinese Academy of Sciences
Tel: 86-64807852
E-mail:ghliu@ioz.ac.cn
Web: http://english.ioz.cas.cn/
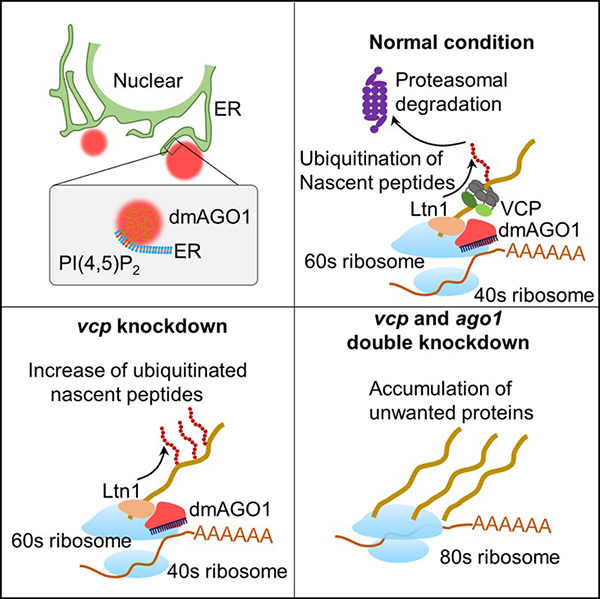
Researchers Reveal a Novel Role of AGO Proteins in Regulating Protein Quality Control on ER
In the recent issue of Molecular Cell (11 a.m. ET on 23 March, 2022), Qinmiao Sun lab from Institute of Stem Cell and Regeneration of the Chinese Academy of Sciences and Dahua Chen lab from Yunnan University discovered that Argonaute proteins (AGOs) on the ER play a novel role in regulating protein quality control via lipid-mediated phase separation, substantially coupling post-transcriptional gene silencing and protein quality control processes to ensure efficient gene silencing.
Mar 23, 2022
The miRNA-mediated gene silencing and the ubiquitin-mediated protein quality control represent two fundamental mechanisms for controlling gene expression. However, little is about how these two processes are coupled to regulate gene expression. In the recent issue of Molecular Cell (11 a.m. ET on 23 March, 2022), Qinmiao Sun lab from Institute of Stem Cell and Regeneration of the Chinese Academy of Sciences and Dahua Chen lab from Yunnan University discovered that Argonaute proteins (AGOs) on the ER play a novel role in regulating protein quality control via lipid-mediated phase separation, substantially coupling post-transcriptional gene silencing and protein quality control processes to ensure efficient gene silencing.
AGOs are well-known to silence target gene expression by interacting with miRNAs and involved in regulating a wide variety of biological processes. In addition to their cytoplasmic localization, AGOs have also been reported to associate with membrane-bound organelles including ER. However, the functional role of AGO proteins' association with the ER membrane and how this association is regulated have been unknown.
In this study, the researchers discovered a conserved lipid-binding motif in the N domain of AGO proteins that specifically interacts with PI(4, 5)P2 and the AGO-PI(4, 5)P2 interaction enhanced the phase separation of AGOs on the ER. Moreover, they found that the ER-localized AGO condensates recruits Ltn1 to catalyze nascent-peptide ubiquitination, subsequently leading to degradation of the unwanted protein products. These findings suggest that AGOs on the ER can couple two fundamental cellular processes, post-transcriptional gene silencing and protein quality control, to ensure proper gene expression.
Given that the human AGO2 overexpression is related to tumorigenesis and cancer aggressiveness and that its lipid-binding motif contains two cancer-related mutations (based on COSMIC database), it will be of great interest to investigate whether the lipid-mediated membrane function of human AGO2 is related to cancer development in the future.
This study entitled "Lipid-mediated phase separation of AGO proteins on the ER controls nascent-peptide ubiquitination" were published in Molecular Cell.
The mechanism of lipid-mediated phase separation of AGO proteins on the ER in regulating nascent-peptide ubiquitination (Image by Chen's lab)
https://www.sciencedirect.com/science/article/pii/S109727652200209X
(11 a.m. ET on 23 March, 2022)
Contact:
Qinmiao Sun
Institute of Zoology Chinese Academy of Sciences
Tel:86-64807391
E-mail: qinmiaosun@ioz.ac.cn
Web: http://english.ioz.cas.cn/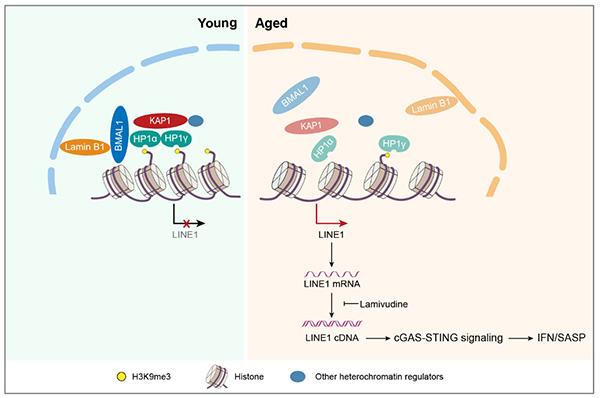
Researchers reveal a new role of circadian protein BMAL1 in antagonizing aging in primates
Recently, researchers from the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences, and Sun Yat-sen University have collaborated to uncover a novel role of BMAL1 in regulating primate stem cell senescence by repressing the“jumping gene” LINE1. This study entitled “BMAL1 moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates” was published online in Nucleic Acids Research on March 14, 2022.
Mar 14, 2022
Circadian rhythms regulate sleep-wake cycles, metabolism, immune function, and reproduction in mammals. These processes are coordinated by the circadian clock, a biochemical oscillator that integrates physiological input signals with distinct oscillatory phases to regulate rhythms in organismal physiology, behavior, and metabolism. Accumulating evidence indicates that aging in mammals is intricately linked with alterations in circadian rhythms. However, whether and how the circadian machinery directly regulates stem cell aging, especially in primates, remains poorly understood.
Recently, researchers from the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences, and Sun Yat-sen University have collaborated to uncover a novel role of BMAL1 in regulating primate stem cell senescence by repressing the“jumping gene” LINE1. This study entitled “BMAL1 moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates” was published online in Nucleic Acids Research on March 14, 2022.
BMAL1, a transcription factor that initiates transcriptional-translational feedback loops that drive the oscillation of circadian genes, is an indispensable component of the molecular circadian. So far, the scientific link between BMAL1 and aging is unclear. In this study, researchers found that nuclear BMAL1 was reduced in senescent mesenchymal progenitor cells (MPCs) from both humans and monkeys, indicating that nuclear BMAL1 might have a conserved function in aging regulation in primates. To study the role of BMAL1 in regulating cellular homeostasis, researchers generated BMAL1-deficient human MPCs by CRISPR/Cas9 gene editing, and found that BMAL1 depletion led to accelerated cellular senescence. Interestingly, the function of BMAL1 in counteracting cellular senescence is independent of its C-terminal transactivation domain and thus uncoupled from its circadian activity. The mechanistic investigation demonstrated that BMAL1 interacted with proteins associated with heterochromatin and nuclear lamina, such as KAP1 and Lamin B1, to stabilize heterochromatin and repress LINE1. BMAL1 deficiency led to detachment of genomic lamina-associated domains (LADs) from the nuclear periphery, decreased H3K9me3 occupancy, and increased chromatin accessibility in LADs.
Further, BMAL1 occupies the LINE1 repetitive elements, and its deficiency causes LINE1 de-repression, which in turn activates cGAS-STING proinflammatory pathways. Importantly, blocking LINE1 activity can alleviate BMAL1 deficiency-induced cellular aging, suggesting that LINE1 repression is likely the mechanism through which BMAL1 regulates senescence. Finally, BMAL1 occupancy at LINE1 was reduced during senescence, and targeted BMAL1 deletion in a monkey model also led to LINE1 activation and tissue aging in vivo, indicating a conserved role of BMAL1 in moonlighting as a gatekeeper for LINE1 repression and cellular senescence in primates.
For the first time, this study identifies a novel function of BMAL1 independent of its transcriptional activity through which it regulates tissue and stem cell senescence. This research expands our understanding of the biological functions of core circadian clock proteins, sheds new light on an inextricable connection between circadian rhythm and aging regulation, and may provide a novel target for the treatment of aging-associated degenerative disorders in the future.
Figure: Dampened BMAL1 occupancy on LINE1 elements leads to its derepression and thus activates cellular senescence through cGAS-STING pathway.
链接:https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkac146/6548307?login=true
( Contact : Guang-Hui Liu , ghliu@ioz.ac.cn )
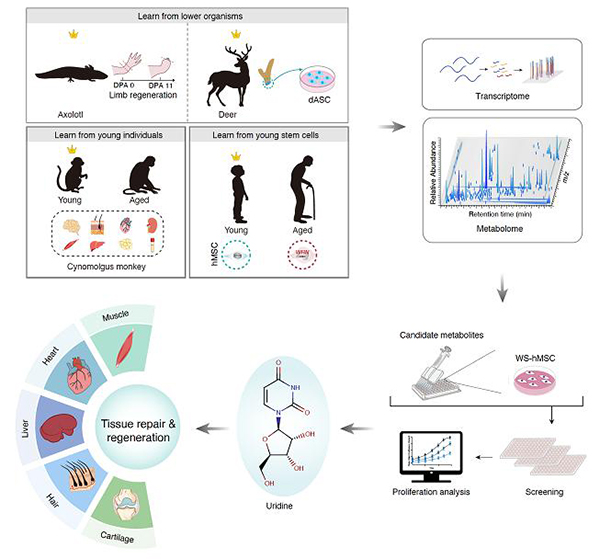
Researchers identify an endogenous metabolite that promotes multiple tissue regeneration and delays aging
Recently, researchers from the Institute of Stem Cell and Regeneration and Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to identify uridine as a pro-regenerative metabolite that promoted human stem cell activity and enhanced regeneration and tissue repair in multiple tissues in mammals. This study entitled “Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor” was published online in Cell Discovery on February, 2022
Feb 01, 2022
Regeneration is an important process of rejuvenating or replacing damaged, diseased, or aged tissues. Regenerative capacity declines with evolution and age. For example, salamanders in lower animals have the complete regenerative capacity in limbs, while in most mammals including humans, limited regeneration and functional recovery capabilities reside in young tissues and decline with age. To date, the molecular mechanism underlying the declined regenerative capacity with evolution and age remains poorly understood. Unlike proteins that are biomacromolecules, the structure of metabolites is relatively similar between species, which makes metabolism an ideal research area for studying evolutionarily conserved biology. Yet, little is known about the small-molecule metabolites that potentially regulate aging and regeneration processes.
Recently, researchers from the Institute of Stem Cell and Regeneration and Beijing Institute of Genomics of the Chinese Academy of Sciences have collaborated to identify uridine as a pro-regenerative metabolite that promoted human stem cell activity and enhanced regeneration and tissue repair in multiple tissues in mammals. This study entitled “Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor” was published online in Cell Discovery on February 1st, 2022.
In this study, by combining metabolomics and transcriptomics approaches, the researchers revealed several cross-species and cross-ages metabolic programs associated with higher regenerative capacity, i.e., polyamine metabolism, pyrimidine metabolism (uracil containing), and fatty acid metabolism pathways. Combined with the human stem cell aging research system, the researchers conducted screening for natural metabolite candidates with regeneration-promoting potentials and identified that uridine counteracted cellular senescence in both physiologically and pathologically senescent cells. Next, the researchers found that uridine supplementation promoted regeneration or repair of various tissues in vivo, including skeletal muscle, heart, liver, skin, and articular cartilage. Specifically, in the muscle injury model, uridine effectively improved muscle regeneration and repair, alleviated the inflammatory response, and endowed uridine-treated mice with higher grip strength and locomotive activity. In the myocardial infarction model, uridine ameliorated acute inflammation and improved the contractility of the injured heart. In the liver fibrosis model, uridine alleviated carbon tetrachloride-induced liver fibrosis and restored the liver function. In the hair regeneration model, uridine induced anagen hair growth. In the arthritis model, uridine promoted the regeneration of articular cartilage and improved grip strength and locomotive activity of mice. In addition, researchers also found that uridine was more abundant in the plasma from young individuals than that from old individuals. Additionally, oral uridine supplementation improved the physical activities of physiologically aged mice.
Taken together, the researchers proved that the supplementation of single metabolite uridine alleviated stem cell senescence, promoted tissue regeneration and repair, and improved the physical activities of aged mice. This study provides previously unknown metabolism-associated regeneration principles across species and opens new avenues for metabolic intervention in tissue repair, regeneration, and aging.
Link: https://www.nature.com/articles/s41421-021-00361-3
Figure legend: Identification of key metabolic small molecules that promote multi-tissue repair and delay aging through cross-species and cross-age metabolome profiling
(Contact: Guang-Hui Liu, ghliu@ioz.ac.cn)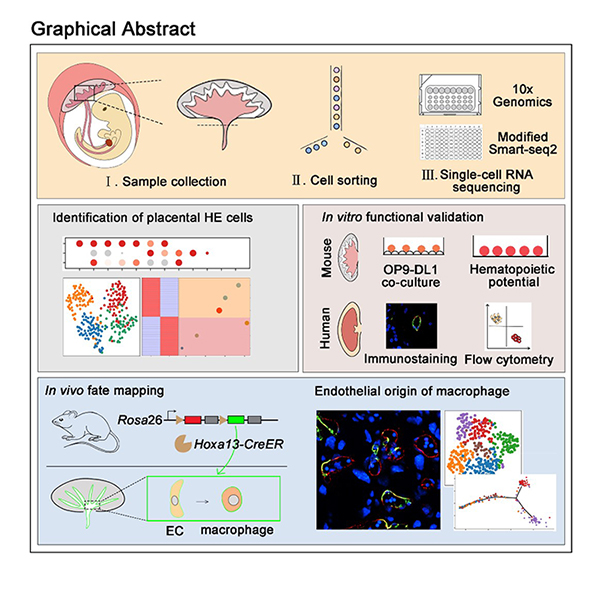
Developmental Cell | De novo generation of macrophage from placenta-derived hemogenic endothelium
On June 30, 2021,Professors Feng Liu (from Institute of Stem Cell and Regeneration, Chinese Academy of Sciences), Jing-Dong J. Han (from Peaking university), Hongmei Wang (from Institute of Stem Cell and Regeneration, Chinese Academy of Sciences) and Berthold Gottgens (from University of Cambridge) collaborate on a paper entitled “De novo generation of macrophage from placenta-derived hemogenic endothelium” in Developmental Cell journal.
Jun 30, 2021
Placenta is a critical organ for embryonic development in the uterus. During pregnancy, the placenta participates in the exchange of gases, nutrients and waste between mother and fetus, secretes hormones and growth factors needed for pregnancy, and protects the fetus from attack by the mother's immune system (Rossant and Cross, 2001). In addition to its role in maternal-fetal communication, placenta has been shown to serve as a source of hematopoietic stem cells during embryogenesis (Gekas et al., 2005; Ottersbach and Dzierzak, 2005). Furthermore, CD41+ hematopoietic cells were observed in the placenta of Ncx1 mutant embryos that lack blood circulation, implying that placenta could generate hematopoietic cells de novo (Rhodes et al., 2008). However, the lethality of Ncx1 mutant in embryos at E11.5 hinders further investigation of the biological function of placenta-resident hematopoietic cells (Koushik et al., 2001).
On June 30, 2021,Professors Feng Liu (from Institute of Stem Cell and Regeneration, Chinese Academy of Sciences), Jing-Dong J. Han (from Peaking university), Hongmei Wang (from Institute of Stem Cell and Regeneration, Chinese Academy of Sciences) and Berthold Gottgens (from University of Cambridge) collaborate on a paper entitled “De novo generation of macrophage from placenta-derived hemogenic endothelium” in Developmental Cell journal. This work identifies and experimentally validates that a CD44+ subpopulation of placental endothelial cells (ECs) exhibits hemogenic potential. Importantly, lineage tracing using the newly-generated Hoxa13 reporter line shows that Hoxa13-labeled ECs can produce placental macrophages, named Hofbauer cell (HBC)-like cells.
They firstly performed 10× Genomics-based single cell RNA sequencing (scRNA-seq) of fetal placental cells and generated a placental cell atlas, including endothelial cells, hematopoietic cells, stromal cells, trophoblast cells and primitive endoderm. They also established an interactive website (http://liulab.ioz.ac.cn/Placenta_hematopoiesis/), with all the data freely available to the community. When focused on placental ECs, all placental ECs were divided into three sub-clusters, annotated as EC1, EC2 and EC3. Among them, they found that EC2 highly expresses genes that are involved in HE specification. Combined with experimental validation, they identified that CD44 can be used to enrich placental HE cells. Transcriptional analysis indicated that Notch and inflammatory signaling are involved in placental HE specification, similar to their reported role in HE specification in aorta-gonad-mesonephros (AGM) region.
Furthermore, they performed scRNA-seq using ECs from yolk sac, AGM and placenta and identified that Hoxa13 is specifically expressed in placental ECs. Then, they established a tamoxifen (TAM)-inducible model (named as Hoxa13CreER) and crossed it with the Rosa26mT/mG reporter line. Lineage tracing using the newly-generated Hoxa13 reporter line showed that ECs give rise to macrophage through placental HE cells. Furthermore, cell-cell interaction analysis predicted the immune response and pro-angiogenic role of placental macrophages.
Taken together, this study generates the atlas of the fetal placenta and provides an important resource for placental biology. This work also extends the potential clinical value of placenta as a new source of HE cells and macrophages. This work was supported by grants from the National Key Research and Development Program of China, the Strategic Priority Research Program of the Chinese Academy of Sciences and the National Natural Science Foundation of China.
References
Gekas, C., Dieterlen-Lievre, F., Orkin, S.H., Mikkola, H.K., 2005. The placenta is a niche for hematopoietic stem cells. Dev Cell 8, 365-375.
Koushik, S.V., Wang, J., Rogers, R., Moskophidis, D., Lambert, N.A., Creazzo, T.L., Conway, S.J., 2001. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15, 1209-1211.
Ottersbach, K., Dzierzak, E., 2005. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell 8, 377-387.
Rhodes, K.E., Gekas, C., Wang, Y., Lux, C.T., Francis, C.S., Chan, D.N., Conway, S., Orkin, S.H., Yoder, M.C., Mikkola, H.K., 2008. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252-263.
Rossant, J., Cross, J.C., 2001. Placental development: lessons from mouse mutants. Nat Rev Genet 2, 538-548.
(Contact: Feng Liu, liuf@ioz.ac.cn)
Tick tock in the brain: Scientists provide molecular insights into primate hippocampal aging
Using brain tissues from non-human primates (NHPs), the ideal model to mimic human hippocampal aging, scientists from the Institute of Stem Cell and Regeneration,CAS and Xuanwu Hospital Capital Medical University have established the first single-nucleus transcriptomic landscape of primate hippocampal aging, revealed the molecular mechanism of its functional deterioration with age...
May 30, 2021
Deep inside our brain is a region called the hippocampus. It plays a crucial role in learning and memory, and its progressive deterioration with age is functionally linked to a variety of human neurodegenerative diseases. But what drives it down the path of aging?
The hippocampus is a complex structure with a highly heterogeneous cell composition, so it is difficult to accurately reveal the molecular regulatory networks of various cell types contributing to the aging process with traditional techniques. In addition, due to the ethical restrictions, it is difficult to obtain disease-free human brain tissues of both young and old ages. All these factors limited our understanding of the aging mechanism in the human hippocampus, let alone the development of therapeutic interventions.
Using brain tissues from non-human primates (NHPs), the ideal model to mimic human hippocampal aging, scientists from the Institute of Stem Cell and Regeneration of the Chinese Academy of Sciences and Xuanwu Hospital Capital Medical University have worked jointly and established the first single-nucleus transcriptomic landscape of primate hippocampal aging, revealed the molecular mechanism of its functional deterioration with age, and provided a valuable resource for the identification of new diagnostic biomarkers and potential therapeutic targets for interventions against hippocampal aging and related human neurodegenerative disorders. This study entitled “Single-nucleus transcriptomic landscape of primate hippocampal aging” is published online in Protein & Cell on May 30, 2021.
In this study, the aged NHP hippocampus was found to demonstrate an array of aging-associated damages, including genomic and epigenomic instability, loss of proteostasis, as well as increased inflammation. To explore unique cellular and molecular characteristics underlying these age-related phenotypes, scientists generated a high-resolution single-nucleus transcriptomic landscape of hippocampal aging in NHPs. This landscape is composed of the gene expression profiles of 12 major hippocampal cell types, including neural stem cells, transient amplified progenitor cells (TAPC), immature neurons, excitatory/inhibitory neurons, oligodendrocytes, and microglia. Among them, TAPC and microglia were most affected by aging, as they manifested the most aging-related differentially expressed genes and those annotated as high-risk genes for neurodegenerative diseases. In-depth analysis of the dynamic gene-expression signatures of the stepwise neurogenesis trajectory revealed the impaired TAPC division and compromised neuronal function, underlying the early onset and later stage of dysregulation in adult hippocampal neurogenesis, respectively. This landscape also enabled us to to unveil contributing factors to a hostile microenvironment for neurogenesis in the aged hippocampus, namely the elevated pro-inflammatory responses in the aged microglia and oligodendrocyte, as well as dysregulated coagulation pathways in the aged endothelial cells. This may aggravate the loss of neurogenesis in the aged hippocampus, and may lead to the further decline of cognitive function and the occurrence of neurodegenerative diseases.
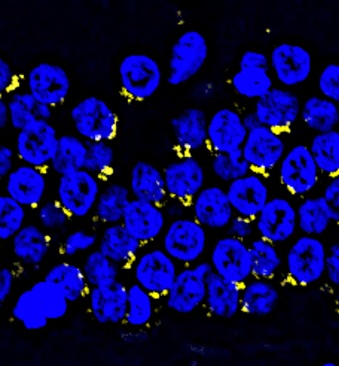
Dangerous protein aggregates (Amyloid-beta) accumulate in the aged monkey hippocampus.
This study established, for the first time, a comprehensive single-nucleus transcriptomic atlas of primate hippocampal aging, which provides extensive resources for the illustration of age-related molecular signatures at the single-cell level, including changes of internal factors and external microenvironment that contribute collectively to the impaired ability for neuronal regeneration in the old hippocampus. It has deepened our understanding of age-related changes in hippocampal structure and function, and identified cell types and molecules that are most susceptible in the aging process of hippocampus, thus enabling the identification of potential diagnostic biomarkers and therapeutic targets for neurodegenerative diseases associated with hippocampal aging.
All relevant data can be accessed via an interactive user-friendly webtool at Aging Atlas (https://bigd.big.ac.cn/aging/index).
This collaborative work was conducted by the Institute of Zoology of the Chinese Academy of Sciences (CAS), the National Clinical Research Center for Geriatric disorders in Xuanwu Hospital Capital Medical University, the Institute of Stem Cell and Regeneration of CAS, Beijing Institute of Genomics of CAS, Peking University Third Hospital, Beijing Institute for Stem Cell and Regenerative Medicine. Hui Zhang, a doctoral candidate at the Institute of Zoology of CAS, Jiaming Li, a graduate student at the Beijing Institute of Genomics of CAS, and Jie Ren, a PI at the Beijing Institute of Genomics of CAS / China national center for bioinformation are the co-first authors. Guang-Hui Liu, a PI at the Institute of Zoology of CAS, Si Wang, a PI at Xuanwu Hospital Capital Medical University, and Jing Qu, a PI at the Institute of Zoology of CAS are the co-corresponding authors. Academician Qi Zhou, Baoyang Hu, and Wei Li, PIs at the Institute of Zoology of CAS, Weiqi Zhang, a PI at the Beijing Institute of Genomics of CAS / China National Center for Bioinformation, Professor Guo-Guang Zhao and Professor Piu Chan from Xuanwu Hospital, and Yang Yu, a PI at Peking University Third Hospital provided important collaboration and support. This work was supported by programs from the Ministry of Science and Technology, the National Natural Science Foundation of China, the Chinese Academy of Sciences and Beijing.
Article Link: https://link.springer.com/article/10.1007/s13238-021-00852-9
(Contact: Guang-Hui Liu, ghliu@ioz.ac.cn)
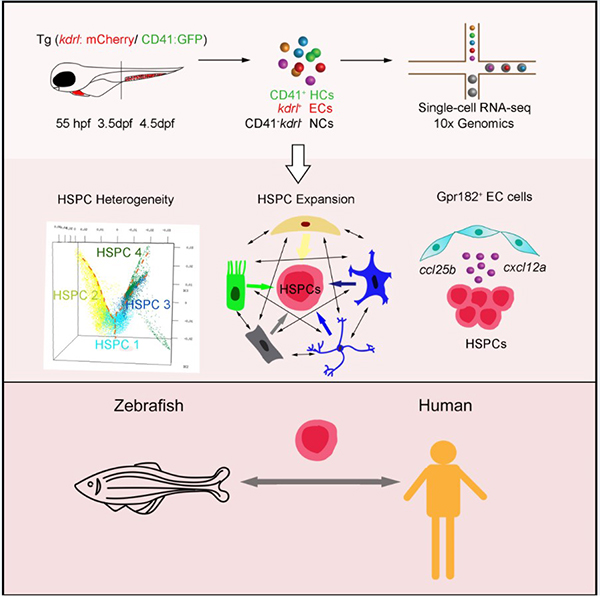
Researchers Provide Novel Insight Into the Hematopoietic Stem and Progenitor Cell Expansion At Single-Cell Resolution
In a recent study published in PNAS, Prof. Feng Liu 's group established the first developmental single-cell transcriptomic atlases of HSPC expansion in zebrafish and provides an essential resource for understanding regulatory mechanisms of HSPC expansion and differentiation in the niche.
Apr 01, 2021
During vertebrate embryogenesis, fetal hematopoietic stem and progenitor cells (HSPCs) exhibit expansion and differentiation properties in a supportive hematopoietic niche (1, 2). Clinically, in vitro HSPC expansion is a feasible approach to obtain sufficient transplantable HSPCs but remains technically challenging (3, 4). Thus, decoding the complex regulatory mechanism of HSPC expansion within the hematopoietic organ is essential.
The caudal hematopoietic tissue (CHT) is characterized as a hematopoietic organ for fetal HSPC expansion in zebrafish (5). CHT is a highly vascularized niche, and the rapidly increased vascular endothelial cells (ECs) form a complex structure composing the dorsal artery and honeycomb-like venous network (6, 7). Therefore, the maintenance of CHT function relies on an orderly and precise process of interaction among all cell types inside (8). However, it remains to be resolved how global niche components orchestrate HSPC development.
Previously, Prof. Feng Liu’s group from the Institute of Stem Cell and Regeneration of the Chinese Academy of Sciences, has revealed the spatiotemporal characteristics of HSPC expansion in the CHT region using bulk RNA-sequencing (RNA-seq) and geographical position sequencing, however, our understanding of molecular and cellular dynamics in CHT hematopoiesis at single-cell resolution remains elusive.
In a recent study published in PNAS, Prof. Feng Liu 's group established the first developmental single-cell transcriptomic atlases of HSPC expansion in zebrafish and provides an essential resource for understanding regulatory mechanisms of HSPC expansion and differentiation in the niche.
The researchers first used single-cell RNA-seq (scRNA-seq) to map the transcriptional profiles of 28,777 cells, which were sorted from the CHT spanning three successive developmental stages. With these profiles, they resolved fetal HSPC heterogeneity, manifested as lineage priming and metabolic gene signatures. Furthermore, they investigated the regulatory mechanism of CHT niche components for HSPC development, with a focus on the transcription factors and ligand–receptor networks involved in HSPC expansion. Importantly, they identified an EC-specific G protein–coupled receptor 182, followed by in vivo and in vitro functional validation of its evolutionally conserved role in supporting HSPC expansion in zebrafish and mice. Finally, comparison between zebrafish CHT and human fetal liver highlighted the conservation and divergence across evolution.
Taken together, the researchers established a high-resolution single-cell transcriptomic atlas of HSPC expansion in the zebrafish CHT. These findings enhanced our understanding of the regulatory mechanism underlying hematopoietic niche for HSPC expansion in vivo and will provide insights into improving protocols for HSPC expansion in vitro.
Figure 1. A single-cell resolution developmental atlas of hematopoietic stem and progenitor cell expansion in zebrafish. (Image by Prof. Feng Liu 's group)
Website: https://doi.org/10.1073/pnas.2015748118
1. E. Laurenti, B. Gottgens, From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418-426 (2018).
2. J. R. Perlin, A. L. Robertson, L. I. Zon, Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J Exp Med 214, 2817-2827 (2017).
3. C. R. Mantel et al., Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell 161, 1553-1565 (2015).
4. A. C. Wilkinson, K. J. Igarashi, H. Nakauchi, Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat Rev Genet 21, 541-554 (2020).
5. S. H. Orkin, L. I. Zon, Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631-644 (2008).
6. E. Murayama et al., Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963-975 (2006).
7. Y. Xue et al., The Vascular Niche Regulates Hematopoietic Stem and Progenitor Cell Lodgment and Expansion via klf6a-ccl25b. Dev Cell 42, 349-362 e344 (2017).
8. Y. Xue et al., A 3D Atlas of Hematopoietic Stem and Progenitor Cell Expansion by Multi-dimensional RNA-Seq Analysis. Cell Rep 27, 1567-1578 e1565 (2019).
(Contact: Prof. Feng Liu, liuf@ioz.ac.cn)

Researchers reveal the roles of protein O-GlcNAcylation in modulating human placental trophoblast differentiation
The research group led by Prof. Yang-Ling Wang at the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences (CAS), in collaboration with Drs. Chu Wang and Xing Chen from Peking University, has now made a major breakthrough about the regulation of trophoblast differentiation by post-translational protein modification through applying the quantitative O-GlcNAc proteomics in human trophoblasts. This study was published in Cell Chemical Biology on Feb. 23, 2021.
Feb 23, 2021
O-linked β-N-acetylglucosamine (O-GlcNAc) is a reversible and ubiquitous post-translational modification in eukaryotic cells, which are extensively involved in a wide range of cellular processes. There has been evidence suggesting the involvement of O-GlcNAcylation in embryonic development or fetal health, whereas the underlying mechanisms remain illusive.
As a temporary yet indispensable organ, the placenta is responsible for intrauterine development of the fetus during pregnancy. At the outermost surface of the placenta, the syncytial layer comprises the multinucleated syncytiotrophoblast (STB) which is formed through cell fusion of the mononucleated cytotrophoblasts (CTB), a process of syncytialization that is primarily induced by the activation of protein kinase A (PKA). STB is directly bathed in maternal blood, thus being positioned to regulate feto-maternal material exchange. Pregnant disorders, such as preeclampsia (PE) and intrauterine growth restriction (IUGR), are in tight association with compromised syncytialization. Thus revealing the regulatory mechanisms of placental trophoblast differentiation is of great value for maternal-fetal health. To date, the dynamic property and working mechanism of O-GlcNAcylation in trophoblast syncytialization remain largely unexplored.
The research group led by Prof. Yang-Ling Wang at the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences (CAS), in collaboration with Drs. Chu Wang and Xing Chen from Peking University, has now made a major breakthrough about the regulation of trophoblast differentiation by post-translational protein modification through applying the quantitative O-GlcNAc proteomics in human trophoblasts. This study was published in Cell Chemical Biology on Feb. 23, 2021.
The researchers employed the chemoenzymatic O-GlcNAc labeling and the reductive dimethylation-based quantitative proteomics to quantify the dynamics of O-GlcNAcylated proteins during in vitro syncytialization of BeWo cells (a human trophoblastic cell line) induced by PKA activator, forskolin. The first global dataset of O-GlcNAcylated proteins from human placental trophoblast cell was established, and hundreds proteins that were dynamically O-GlcNAcylated during trophoblast syncytialization were identified.
Among those PKA-induced GlcNAcylated proteins, Cystathionine γ-lyase (CSE) exhibited the most significant change. By applying the methods of click chemistry and quantitative O-GlcNAc stoichiometry, it was revealed that CSE O-GlcNAcylation in trophoblasts was a very early response to PKA and exerted steady-going influence on CSE function. Site-specific analysis by mass spectrometry revealed Ser138 as the core O-GlcNAc site in CSE, and its O-GlcNAcylation promoted the enzymatic activity of CSE to produce H2S. The GlcNAcylated CSE-boosted H2S efficiently inhibited androgen receptor (AR) dimerization and thus hampered testosterone-enhanced trophoblast syncytialization.
The pathological relevance of O-GlcNAcylation in pregnancy complication was revealed in the placentas from severe preeclamptic patients which displayed remarkably enhanced CSE O-GlcNAcylation and H2S production, as well as restricted trophoblast differentiation.
In general, the multidisciplinary approach in this study provides a resource of O-GlcNAc dynamics in human placenta and uncovers a key role of CSE O-GlcNAcylation in controlling trophoblast differentiation. The finding reveals a guarding mechanism CSE O-GlcNAcylation to control an appropriate degree of syncytialization through coordinating PKA and AR signals. The study make a deeper insight into the biological significance of O-GlcNAcylation in placental development as well as the potential therapeutic targets for the placenta-associated pregnant complications.
The working model of cystathionine γ-lyase (CSE) O-GlcNAcylation to modulate trophoblast syncytialization.
Contact
Yan-Ling Wang
Institute of Zoology, Chinese Academy of Sciences
E-mail: wangyl@ioz.ac.cn
http://english.rpb.ioz.cas.cn/groups/wangyanling/
Reference
Quantitative Chemoproteomics Reveals O-GlcNAcylation of Cystathionine γ-lyase (CSE) Represses Trophoblast Syncytialization.
https://doi.org/10.1016/j.chembiol.2021.01.024

