Newsroom
Science Update
Researchers reveal the roles of protein O-GlcNAcylation in modulating human placental trophoblast differentiation
Feb 23, 2021
O-linked β-N-acetylglucosamine (O-GlcNAc) is a reversible and ubiquitous post-translational modification in eukaryotic cells, which are extensively involved in a wide range of cellular processes. There has been evidence suggesting the involvement of O-GlcNAcylation in embryonic development or fetal health, whereas the underlying mechanisms remain illusive.
As a temporary yet indispensable organ, the placenta is responsible for intrauterine development of the fetus during pregnancy. At the outermost surface of the placenta, the syncytial layer comprises the multinucleated syncytiotrophoblast (STB) which is formed through cell fusion of the mononucleated cytotrophoblasts (CTB), a process of syncytialization that is primarily induced by the activation of protein kinase A (PKA). STB is directly bathed in maternal blood, thus being positioned to regulate feto-maternal material exchange. Pregnant disorders, such as preeclampsia (PE) and intrauterine growth restriction (IUGR), are in tight association with compromised syncytialization. Thus revealing the regulatory mechanisms of placental trophoblast differentiation is of great value for maternal-fetal health. To date, the dynamic property and working mechanism of O-GlcNAcylation in trophoblast syncytialization remain largely unexplored.
The research group led by Prof. Yang-Ling Wang at the Institute of Stem Cell and Regeneration, Chinese Academy of Sciences (CAS), in collaboration with Drs. Chu Wang and Xing Chen from Peking University, has now made a major breakthrough about the regulation of trophoblast differentiation by post-translational protein modification through applying the quantitative O-GlcNAc proteomics in human trophoblasts. This study was published in Cell Chemical Biology on Feb. 23, 2021.
The researchers employed the chemoenzymatic O-GlcNAc labeling and the reductive dimethylation-based quantitative proteomics to quantify the dynamics of O-GlcNAcylated proteins during in vitro syncytialization of BeWo cells (a human trophoblastic cell line) induced by PKA activator, forskolin. The first global dataset of O-GlcNAcylated proteins from human placental trophoblast cell was established, and hundreds proteins that were dynamically O-GlcNAcylated during trophoblast syncytialization were identified.
Among those PKA-induced GlcNAcylated proteins, Cystathionine γ-lyase (CSE) exhibited the most significant change. By applying the methods of click chemistry and quantitative O-GlcNAc stoichiometry, it was revealed that CSE O-GlcNAcylation in trophoblasts was a very early response to PKA and exerted steady-going influence on CSE function. Site-specific analysis by mass spectrometry revealed Ser138 as the core O-GlcNAc site in CSE, and its O-GlcNAcylation promoted the enzymatic activity of CSE to produce H2S. The GlcNAcylated CSE-boosted H2S efficiently inhibited androgen receptor (AR) dimerization and thus hampered testosterone-enhanced trophoblast syncytialization.
The pathological relevance of O-GlcNAcylation in pregnancy complication was revealed in the placentas from severe preeclamptic patients which displayed remarkably enhanced CSE O-GlcNAcylation and H2S production, as well as restricted trophoblast differentiation.
In general, the multidisciplinary approach in this study provides a resource of O-GlcNAc dynamics in human placenta and uncovers a key role of CSE O-GlcNAcylation in controlling trophoblast differentiation. The finding reveals a guarding mechanism CSE O-GlcNAcylation to control an appropriate degree of syncytialization through coordinating PKA and AR signals. The study make a deeper insight into the biological significance of O-GlcNAcylation in placental development as well as the potential therapeutic targets for the placenta-associated pregnant complications.
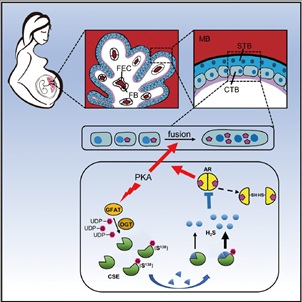
The working model of cystathionine γ-lyase (CSE) O-GlcNAcylation to modulate trophoblast syncytialization.
Contact
Yan-Ling Wang
Institute of Zoology, Chinese Academy of Sciences
E-mail: wangyl@ioz.ac.cn
http://english.rpb.ioz.cas.cn/groups/wangyanling/
Reference
Quantitative Chemoproteomics Reveals O-GlcNAcylation of Cystathionine γ-lyase (CSE) Represses Trophoblast Syncytialization.
https://doi.org/10.1016/j.chembiol.2021.01.024
附件:

